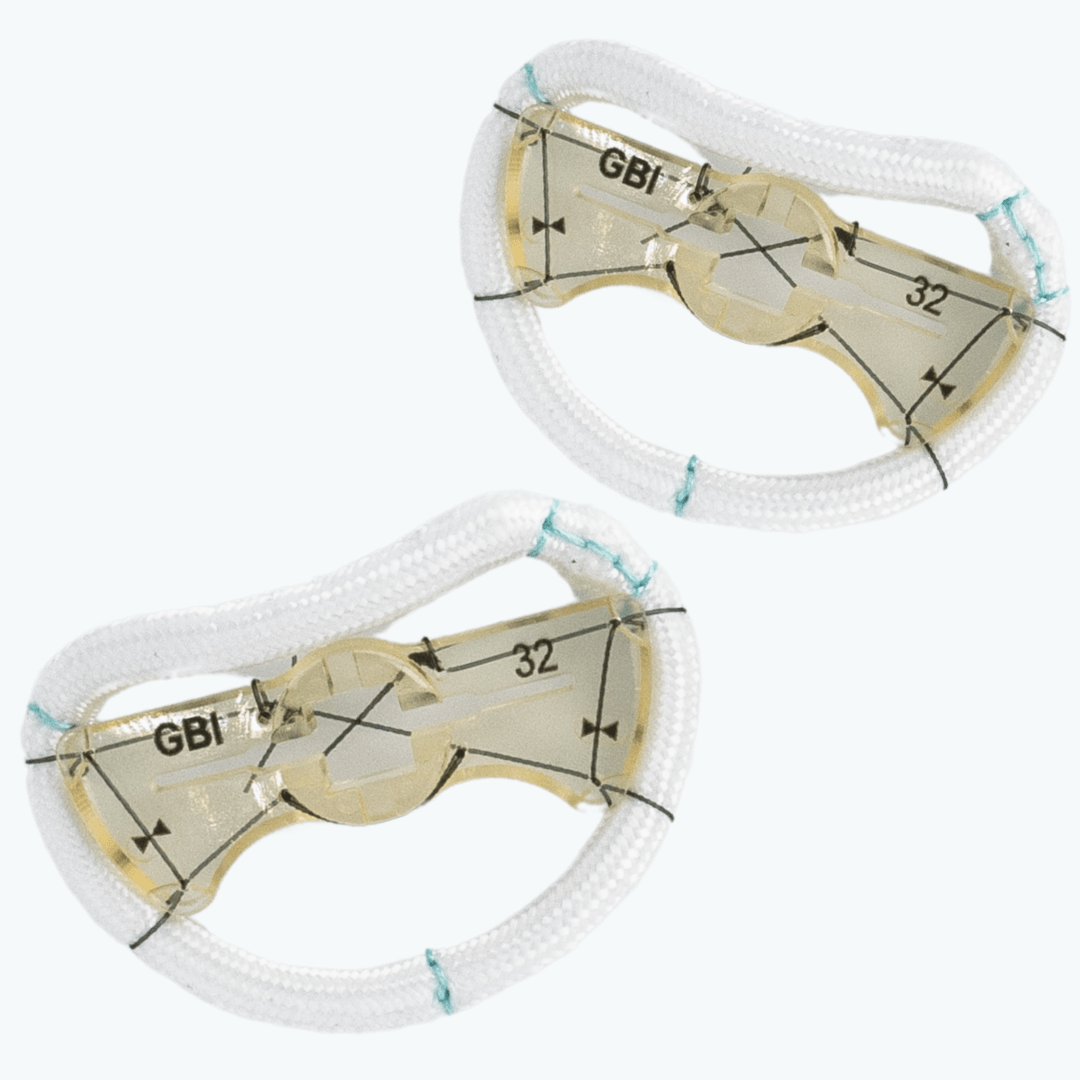
TruForm™ Sievers Annuloplasty Ring
Semi-Rigid Strength for Precise Mitral Repair. True Physiological Repair.
Semi-Rigid, Designed for Consistency Across the Cardiac Cycle.
TruForm™ is a semi-rigid mitral annuloplasty ring engineered to provide stable, anatomical support while preserving physiologic annular motion. Designed with surgical landmarks and adjacent structures in mind, it offers a precise anatomical fit — making it ideal for surgeons focused on consistency and durability across the cardiac cycle.
Key Features:
-
-
Surgical reconstruction or remodeling of the mitral valve
-
Procedures requiring durable, semi-rigid annular support
-
Surgeons who prefer a ring congruent with surgical landmarks
-
Anatomies involving the trigones, LVOT, and aortic annulus
-
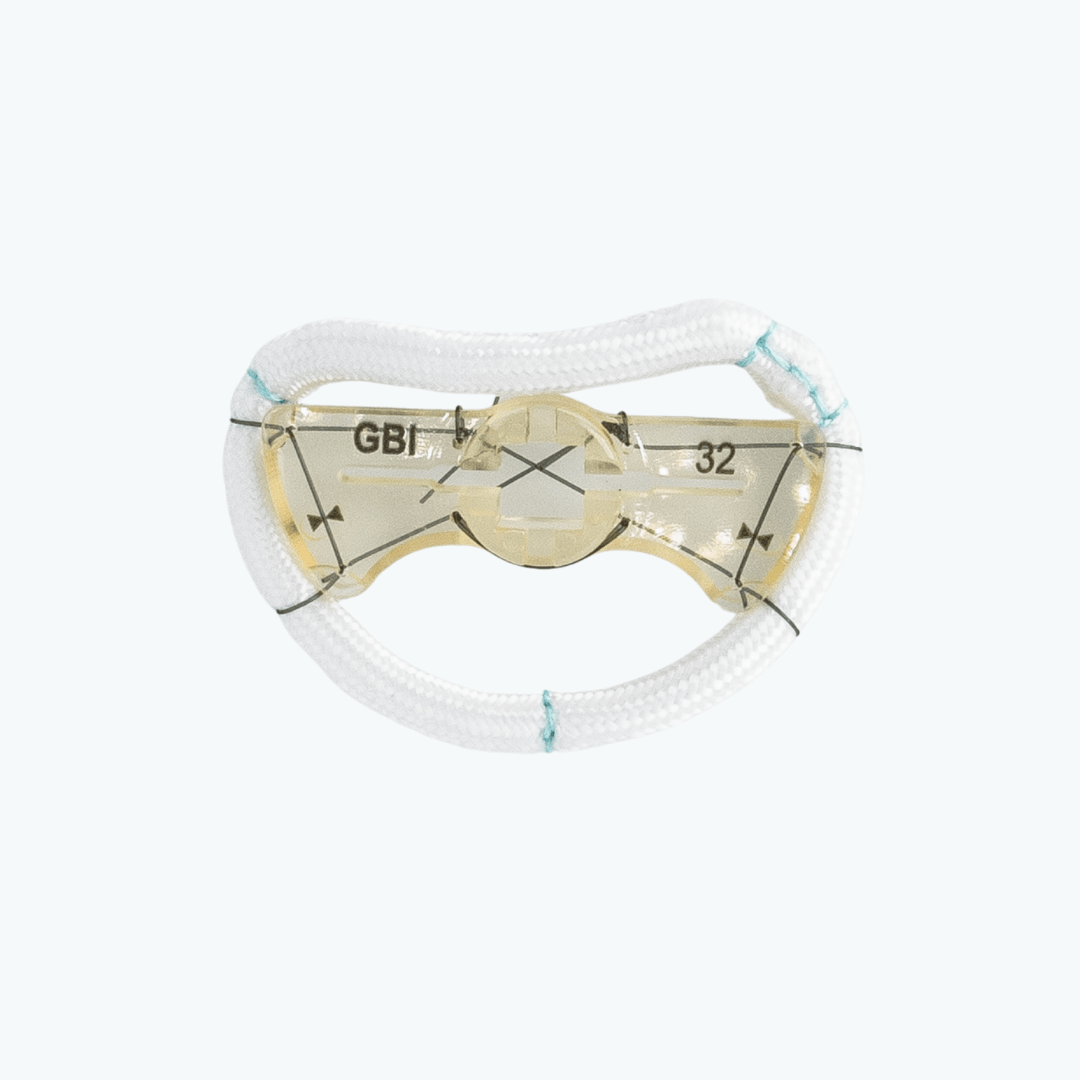
Clinical Considerations
The TruForm™ ring supports mechanical stress reduction through annular stabilization while preserving natural annular motion. Its semi-rigid design helps maintain valve competence without over-constraining the anatomy. Available in both complete and posterior-only configurations, TruForm™ provides options for enhanced visualization and flexibility depending on surgical preference. It is especially suited for anatomies requiring precise alignment with adjacent cardiac structures.
Ready to learn more?
Balancing Flexibility and Rigidity for Optimal Valve Repair
TruForm™ offers a semi-rigid ring that mirrors the natural shape of the mitral annulus while aligning with surgical landmarks for effective implantation and physiological support.
This balance of stability and anatomical conformity helps maintain mitral competence throughout the cardiac cycle — providing surgeons with a reliable and reproducible option for durable repair.
TruForm™ Sievers Annuloplasty Ring Video
Ordering Information
TruForm™ Sievers Annuloplasty Ring
| Catalog # | Product Description |
|---|---|
| TRH‑24 | TruForm™ Annuloplasty Ring size 24 |
| TRH‑26 | TruForm™ Annuloplasty Ring size 26 |
| TRH‑28 | TruForm™ Annuloplasty Ring size 28 |
| TRH‑30 | TruForm™ Annuloplasty Ring size 30 |
| TRH‑32 | TruForm™ Annuloplasty Ring size 32 |
| TRH‑34 | TruForm™ Annuloplasty Ring size 34 |
| TRH‑36 | TruForm™ Annuloplasty Ring size 36 |
| TRH‑38 | TruForm™ Annuloplasty Ring size 38 |
| TRH‑40 | TruForm™ Annuloplasty Ring size 40 |
| TRH‑42 | TruForm™ Annuloplasty Ring size 42 |
Accessory Ordering Information – TruForm™ Annuloplasty Ring
Accessories are sold separately. Order using the model numbers.
| Catalog # | Accessory Description |
|---|---|
| GSS‑26/28 | Stainless Steel Sizer, double-ended 26/28 |
| GSS‑30/32 | Stainless Steel Sizer, double-ended 30/32 |
| GSS‑34/36 | Stainless Steel Sizer, double-ended 34/36 |
| GSS‑38/40 | Stainless Steel Sizer, double-ended 38/40 |
| GSS‑4 | Stainless Steel Sizer, double-ended set of 4 |
| FRBS | Flexible Silicone Sizers, set of 9 (Sizes 24–40) |
| GRH‑U | Reusable Handle |
| GRH‑UXL | Extra-Long Reusable Handle (35 cm) |
Get More Information or Connect With a Local Rep
Want to learn more about the TruForm™ Annuloplasty Ring or speak directly with a representative in your area? Fill out the form below and a member of our team will contact you with product details, clinical resources, or to help you schedule a consultation.
By submitting this form, you are consenting to receive marketing emails from: Genesee BioMedical Inc. You can revoke your consent to receive emails at any time by using the SafeUnsubscribe® link, found at the bottom of every email.
Explore Our Complete Cardiac Portfolio
Indications: The TruForm™ Annuloplasty Rings are indicated for the surgical reconstruction or remodeling of diseased or damaged mitral valves. The ring provides support for and restricts expansion of the mitral annulus.
Warnings: Only surgeons who have received adequate training to determine whether incompetent, stenotic, or diseased heart valves are capable of being repaired or replaced should use this device. Refer to the Instructions for Use (IFU) for a full list of warnings, precautions and adverse events.
CAUTION: Federal law (USA) restricts this device to sale by, or on the order of, a physician.
Image Credits: Images courtesy of Dr. Kevin Miller, Dr. Steven Bolling, and Dr. Jose Navia.
Design Beyond Standard
303-777-3000
800-786-4890
700 W. Mississippi Ave. #D5
Denver CO 80223 – 4509